Speeding up scientific computing with multiprocessing in Python
A real-world example of how multiprocessing can drastically speed up scientific computations
Photo by Christian Wiediger on Unsplash
In this tutorial, we will look at how we can speed up scientific computations using multiprocessing in a real-world example. Specifically, we will detect the location of all nuclei within fluorescence microscopy images from the public MCF7 Cell Painting dataset released by the Broad Institute.
After completing this tutorial, you will know how to do the following:
- Detect nuclei in fluorescence microscopy images
- Speed up image processing using
multiprocessing.Pool - Reduce serialization overhead using copy-on-write (Unix only)
- Add a progress bar to parallel processing tasks
Download the dataset
Note: The imaging data is nearly 1 GB for just one plate. You will need a few GB of space to complete this tutorial.
First, download the imaging data for the first plate of the Cell Painting dataset here to a folder called images/ and extract it. You should get a folder called images/Week1_22123/ filled with TIFF images. The images are named according to the following convention:
# Template
{week}_{plate}_{well}_{field}_{channel}{id}.tif
# Example
Week1_150607_B02_s4_w1EB868A72-19FC-46BB-BDFA-66B8966A817C.tif
Find all DAPI images
DAPI is a fluorescent dye that binds to DNA and will stain the nuclei in each image. Typically, DAPI signal will be captured in the first channel of the microscope. We'll first need to parse the image names to find images from the first channel (w1) so that we can detect the nuclei from only the DAPI images.
from glob import glob
paths = glob('images/Week1_22123/Week1_*_w1*.tif')
paths.sort()
print(len(paths)) # => 240
Here, we used glob and a pattern with wildcards to find the paths to all DAPI images. Apparently there are 240 DAPI images that we need to process! We can load the first image to see what we are working with.
from skimage import io
import matplotlib.pyplot as plt
img = io.imread(paths[0])
print(img.shape, img.dtype, img.min(), img.max())
# => (1024, 1280) uint16 176 10016
plt.figure(figsize=(6, 6))
plt.imshow(img, cmap='gray', vmax=4000)
plt.axis('off')
plt.show()
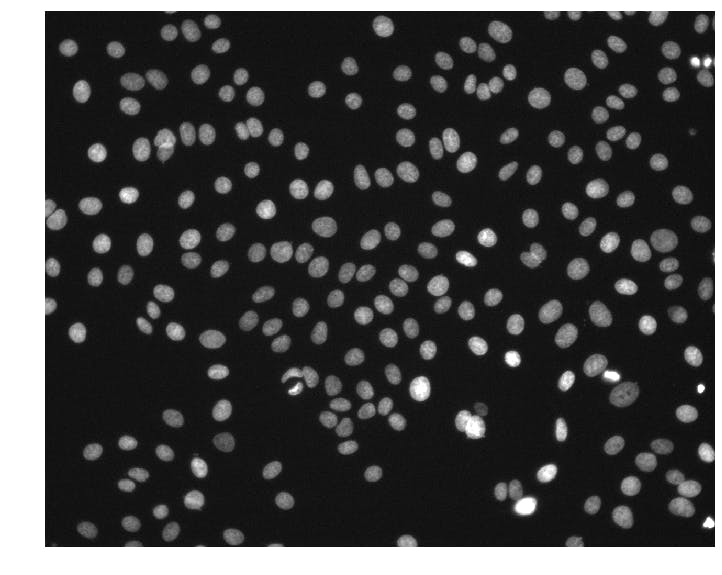
We can see that the images are (1024, 1280) arrays of 16-bit unsigned integers. This particular image has pixel intensities ranging from 176 to 10,016.
Using skimage to detect nuclei
Scikit-image is a Python package for image processing that we can use to detect nuclei in these DAPI images. A simple and effective approach is to smooth the image to reduce noise and then find all local maxima that are above a given intensity threshold. Let's write a function to do this on our example image.
from skimage.filters import gaussian
from skimage.feature import peak_local_max
def detect_nuclei(img, sigma=4, min_distance=6, threshold_abs=1000):
g = gaussian(img, sigma, preserve_range=True)
return peak_local_max(g, min_distance, threshold_abs)
centers = detect_nuclei(img)
print(centers.shape) # => (214, 2)
plt.figure(figsize=(6, 6))
plt.imshow(img, cmap='gray', vmax=4000)
plt.plot(centers[:, 1], centers[:, 0], 'r.')
plt.axis('off')
plt.show()
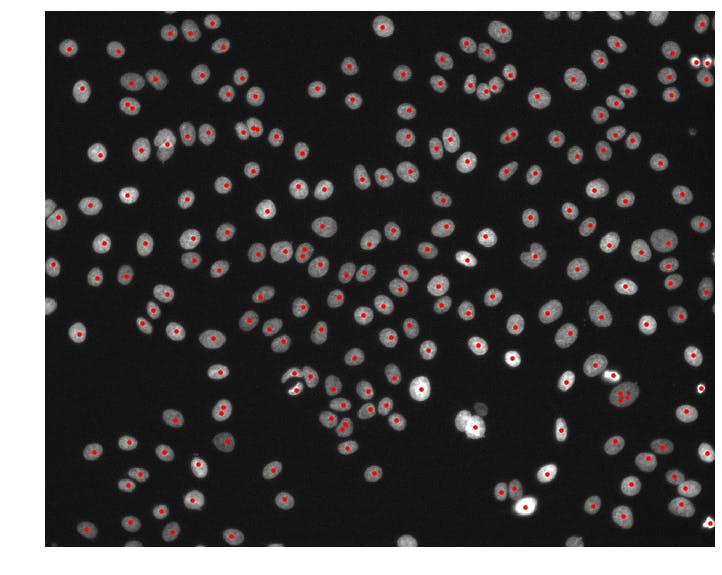
Now we're getting somewhere! The detect_nuclei function takes in an img array and returns an array of nuclei (x, y)-coordinates called centers. It first smooths the img array by applying a gaussian blur with sigma = 4. The preserve_range = True argument prevents skimage from normalizing the image so that we get back a smoothed image g in the same intensity range as the input. Then, we use the peak_local_max function to detect all local maxima in our smoothed image. We set min_distance = 4 to prevent any two nuclei centers from being unrealistically close together and threshold_abs = 1000 to avoid detecting false positives in dark regions of the image.
Note: We will use this
detect_nucleifunction in all subsequent experiments.
Method 1 - List comprehension with IO
Now that we have a function to detect nuclei coordinates, let's apply this function to all of our DAPI images using a simple list comprehension.
from tqdm.notebook import tqdm
def process_images1(paths):
return [detect_nuclei(io.imread(p)) for p in paths]
meth1_times = %timeit -n 4 -r 1 -o centers = process_images1(tqdm(paths))
# => 18 s ± 0 ns per loop (mean ± std. dev. of 1 run, 4 loops each)
In process_images1, we take in a list of image paths and use a list comprehension to load each image and detect nuclei. Using the %timeit magic command in Jupyter, we can see that this approach has an average execution time of ~18 seconds.
Method 2 - multiprocessing.Pool with IO
Let's see if we can speed things up with a processing Pool. To do this, we need to define a function to wrap detect_nuclei and io.imread together so that we can map that function over the list of image paths.
import multiprocessing as mp
def _process_image(path):
return detect_nuclei(io.imread(path))
def process_images2(paths):
with mp.Pool() as pool:
return pool.map(_process_image, paths)
meth2_times = %timeit -n 4 -r 1 -o centers = process_images2(paths)
# => 5.54 s ± 0 ns per loop (mean ± std. dev. of 1 run, 4 loops each)
Much better. This was run on a machine with cpu_count() == 8. Now the same task has an average execution time of ~5.5 seconds (>3x speed up).
Method 3 - List comprehension from memory
What would happen if we read all the images into memory before detecting nuclei? Presumably, this approach would be faster because we wouldn't have to read the image data from disk during the %timeit command. Let's try it.
import numpy as np
images = np.asarray([io.imread(p) for p in tqdm(paths)])
def process_images3(images):
return [detect_nuclei(img) for img in images]
meth3_times = %timeit -n 4 -r 1 -o centers = process_images3(tqdm(images))
# => 17.7 s ± 0 ns per loop (mean ± std. dev. of 1 run, 4 loops each)
Now the list comprehension is slightly faster, but only by about 0.3 seconds. This suggests that reading image data is not the rate-limiting step in Method 1, but rather the detect_nuclei computations.
Method 4 - multiprocessing.Pool from memory
Just for completeness, let's try using multiprocessing.Pool to map our detect_nuclei function over all the images in memory.
def process_images4(images):
with mp.Pool() as pool:
return pool.map(detect_nuclei, images)
meth4_times = %timeit -n 4 -r 1 -o centers = process_images4(images)
# => 6.23 s ± 0 ns per loop (mean ± std. dev. of 1 run, 4 loops each)
Wait, what? Why is this slower than also reading the images from disk with multiprocessing? If you read the documentation for the multiprocessing module closely, you will learn that data passed to workers of a Pool must be serialized via pickle. This serialization step creates some computational overhead. That means for Method 2, we were pickling path strings, but in this case, we were pickling entire images.
Method 5 - multiprocessing.Pool with serialization fix
Fortunately, there are several ways to avoid this serialization overhead when using multiprocessing. On Mac and Linux systems, we can take advantage of how the operating system handles process forks to efficiently process large arrays in memory (sorry Windows 🤷♂️). Unix-based systems use copy-on-write behavior for forked processes. Loosely, copy-on-write means the forked process will only copy data when attempting to modify the shared virtual memory. This all happens behind the scenes, and copy-on-write is sometimes referred to as implicit sharing.
def _process_image_memory_fix(i):
global images
return detect_nuclei(images[i])
def process_images5(n):
with mp.Pool() as pool:
return pool.map(_process_image_memory_fix, range(n))
meth5_times = %timeit -n 4 -r 1 -o centers = process_images5(len(paths))
# => 5.31 s ± 0 ns per loop (mean ± std. dev. of 1 run, 4 loops each)
Nice! This is now slightly faster than Method 2 (as we may have originally expected). Notice that we use a global statement here to explicitly declare that this function uses the images array defined globally. While this is not strictly required, I have found it helpful to indicate that the _process_image_memory_fix function depends on some images array being available. We then map over all indices and allow each process to access the image it needs by indexing into the images array. This approach will only pickle integers instead of the images themselves.
Results
Let's compare the average execution times of all 5 methods.
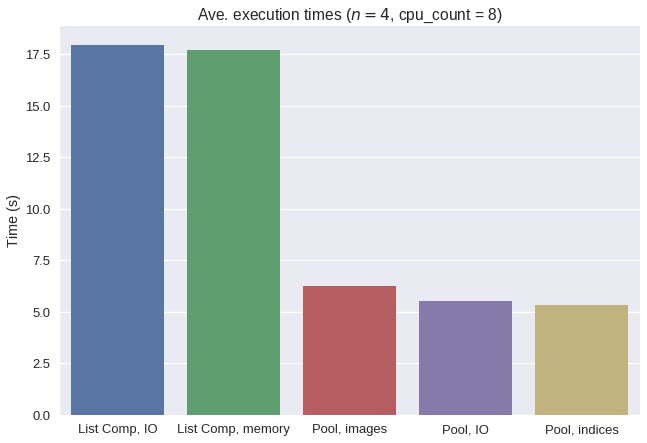
Overall, multiprocessing drastically reduced the average execution times for this nuclei detection task. Interestingly, a naive approach to multiprocessing with images in memory actually increased the average execution time. By taking advantage of copy-on-write behavior, we were able to remove the significant serialization overhead of incurred when pickling whole images.
To show off more of our results, let's show a random sample of 16 DAPI images sorted by the number of detected nuclei in each image.
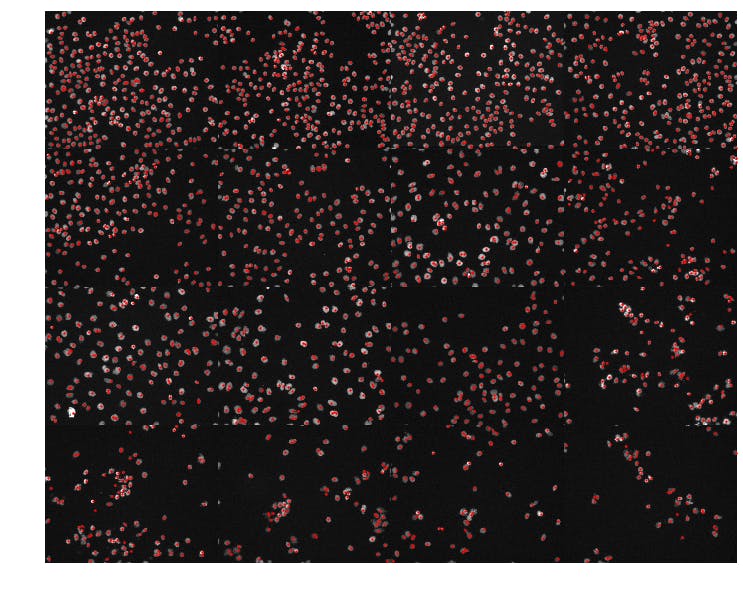
It appears that our simple nuclei detection strategy was effective and has allowed us to organize the DAPI images according to the observed cell density in each field of view.
Conclusion
 From Spaceballs the movie
From Spaceballs the movie
I really hope this article was helpful, so please let me know if you enjoyed it. The multiprocessing techniques presented here have helped me achieve ridiculous speed when processing images for our SCOUT paper. Perhaps in the future we can revisit this example but with GPU acceleration to finally achieve ludicrous speed.
Bonus: Using progress bars with multiprocessing.Pool
In the above multiprocessing examples, we do not have a nice tqdm progress bar. We can get one if we use pool.imap instead, which is useful for long-running computations.
def _process_image(path):
return detect_nuclei(io.imread(path))
def process_images_progress(paths):
with mp.Pool() as pool:
return list(tqdm(pool.imap(_process_image, paths), total=len(paths)))
centers = process_images_progress(paths)
References
We used image set BBBC021v1 [Caie et al., Molecular Cancer Therapeutics, 2010], available from the Broad Bioimage Benchmark Collection [Ljosa et al., Nature Methods, 2012].
Source Availability
All source materials for this article are available here on my blog GitHub repo.

